Which of the Following Does Not Obey Octet Rule
The 3 electrons are of Boron atom and remaining 3 electrons are of 3 fluorine atoms. 35 How many of the following molecule does not follow octet rule.

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube
3 How does oxygen achieve octet.

. Hence it does not obey the octet rule completely. Answers 3 Templates let you quickly answer FAQs or store snippets for re-use. The correct option is B b and c both.
Since carbon is located in period 2 it does not have access to the d sublevel and must adhere to the octet ruleThere are three different possible resonance structures from carbonate. LiCl molecule disobeys octet rule due to incomplete octet. 6 Does CH4 obey the octet rule.
Boron does not always obey the octet rule and in fact forms Lewis acids such as BF3 which only has 6. In this structure it does not follow octet rule because central boron atom is surrounded by 6 electrons. Which of the following contains an atom that does NOT obey the octet rule.
9 How does oxygen bond with itself. 11 When O2 forms How does each. Hydrogen does not obey the octet rule.
Boron has a valence for 6 electrons. Which of the following molecule does NOT obey octet rule. BeC128F3 SF6 SF2 NOCIO2PF5.
While discussing octet rule one does not consider d or f electrons. The most commonly encountered stable species that exist with an odd number of electrons are nitrogen oxides such as nitric oxide NO and nitrogen dioxide NO 2 both of which are free radicals. Compounds following the octet rule must have 8 electrons in the valence shell of.
2 How does O obey the octet rule. The unstable atoms generally lose or gain electrons to obtain the stable configuration of the nearest noble gas. ABH3 BNH3 CPH3 DH2S EAll Of These Obey The Octet Rule.
It is the compound which does not follow the octet rule for electron distribution c SF4 4 dots on the I. Therefore they violate the octet rule. It is because the rule makes it mandatory to have eight electrons around each of the atoms.
The octet rule states that elements will gain or lose electrons in order to have a full outer shell of eight electrons. I believe a possible Lewis dot structure that does not obey the octet rule. Which of the following molecule does NOT obey octet rule.
Furthermore does co32 obey the octet rule. In the given question in BrF3 fluorine follows octet rule while bromine does not. 8 Does oxygen need an octet.
7 Does HCN obey octet rule. State which quantities are scalar and which quantities are vector. Correct options are A B C and D The octet rule states that atoms tend to form compounds in ways that give them eight valence electrons and thus the electron configuration of a noble gas.
As we know Boron lacks an electron pair. The answer above me is most definitely wrong. Explaination Expanded octet can be seen in atoms that have vacant orbital this is an essential requireme.
Which of the following molecules does not obey the octet rule. Solved Which Of The Following Molecules Does Not Obey The Octet Rule A Mathrm Hcn B Mathrm Pf 3 C Mathrm Cs 2 D Mathrm No E None Of These. The central atom B only has 6 electrons surrounding it.
So only the octet of oxygen atom is achieved. Hence the correct answer is option d. Whereas in NH3 and H2O there are 1 and 2 lone pair of electrons respectively on central atom.
Hydrogen and helium are special cases that do not follow the octet rule but the duplet rule. The octet rule states that the states 8 electrons surround the central atom. Molecules having an odd number of electrons around them do not follow the octet rule.
Which one of the following compounds does not follow the octet rule. In CH4 and PCl5 there are no lone pair of electrons on central atom. 5 How does oxygen not follow the octet rule.
10 Why is oxygen more reactive than sulfur. Which of the following contains an atom that does NOT obey the octet rule. Octet rule states that eight electrons in the outermost shell impart stability as in the case of Noble gases.
In BCl3 and P Cl5 B and P contain 6 and 10 electrons respectively in their valence shell. Experts are tested by Chegg as specialists in. Who are the experts.
Which molecule has a Lewis structure that does not obey the octet rule. Having an odd number of electrons in a molecule guarantees that it does not follow the octet rule because the rule requires eight electrons or two for hydrogen around each atom. 4 Does 02 obey the octet rule.
So to satisfy octet rule there is in need 8 electrons which follow the stable structure. The atomic number of Lithium is 3 and the electronic configuration is. When metal carbonates are heated strongly metal oxides are formed.
According to the octet rule the atoms of the prime-group elements seem to bind in such a manner that each atom exhibit eight electrons in its valence shell providing it the similar electronic configuration as a noble gas. Click hereto get an answer to your question Question. Sulfur phosphorus silicon and chlorine are common examples of elements that form an expanded octet.
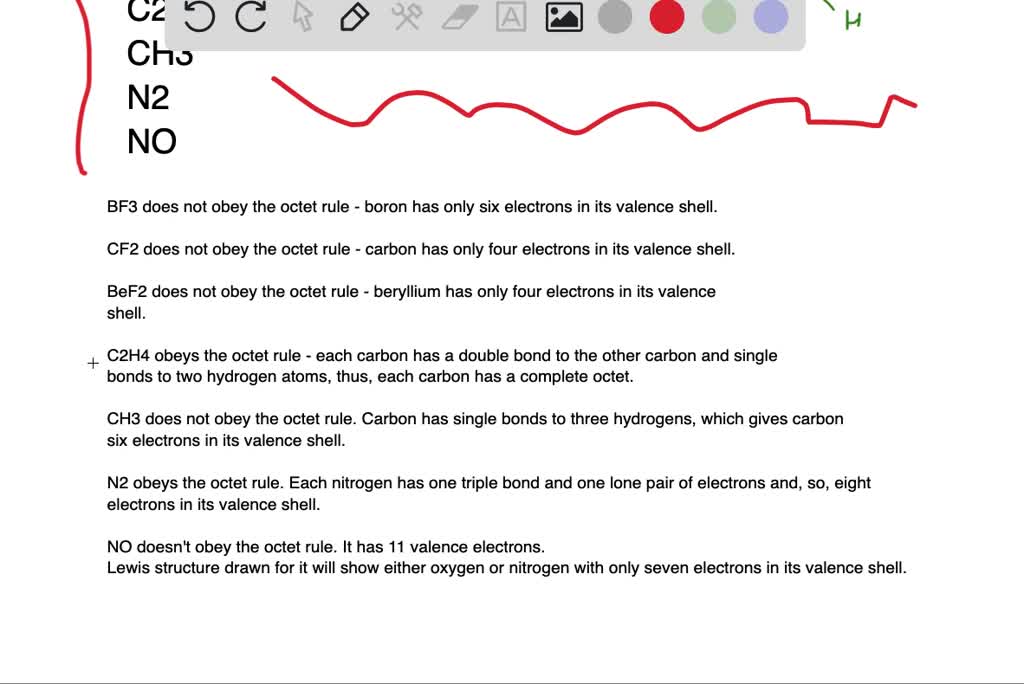
Solved Which Of The Following Molecules Have An Atom That Does Not Obey The Octet Rule Not All Of These Are Stable Molecules A B F 3 B Mathrm Cf 2 C Operatorname Be Mathrm F 2 D Mathrm C 2

Which Of The Following Compound Obey Octet Rule Youtube

Which Of The Following Does Not Follow The Octet Rule

Solved Which Of The Molecules Does Not Obey The Octet Rule Chegg Com

Which Of The Following Does Not Follow The Octet Rule Youtube

Solved Which Of The Following Does Not Obey The Octet Rule Chegg Com

Which Of The Following Does Not Follow The Octet Rule
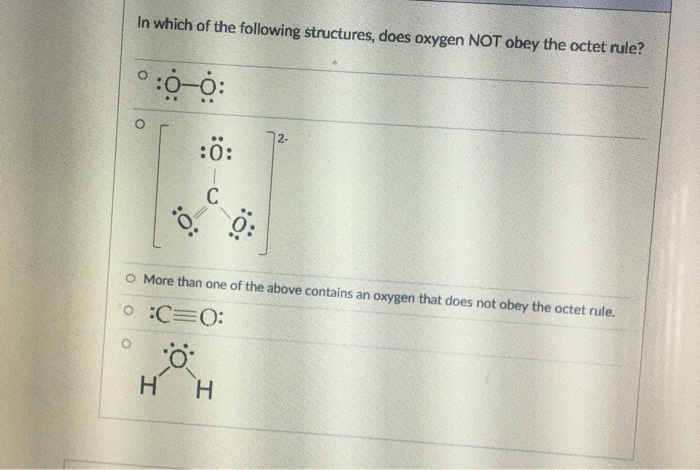
Solved In Which Of The Following Structures Does Oxygen Not Chegg Com
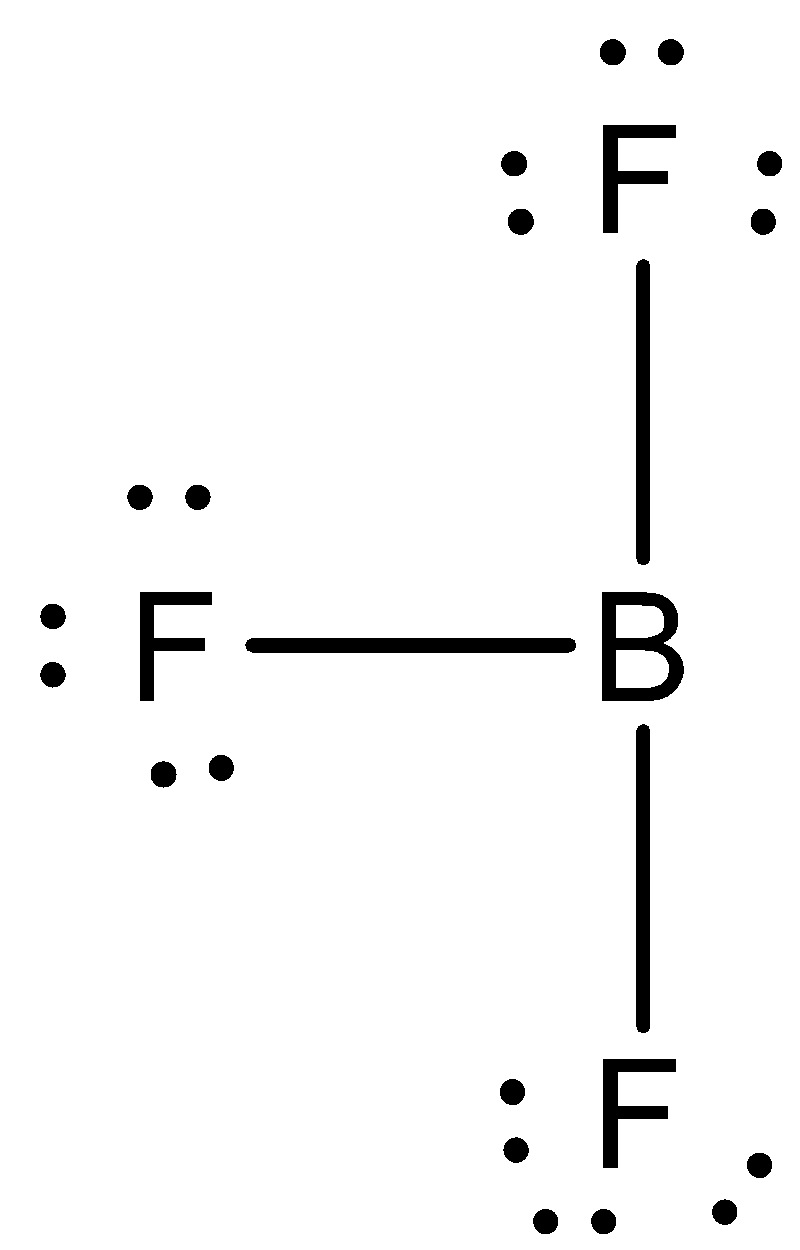
Bf3 Does Not Obey Octet Rule State The Given Statement Class 11 Chemistry Cbse
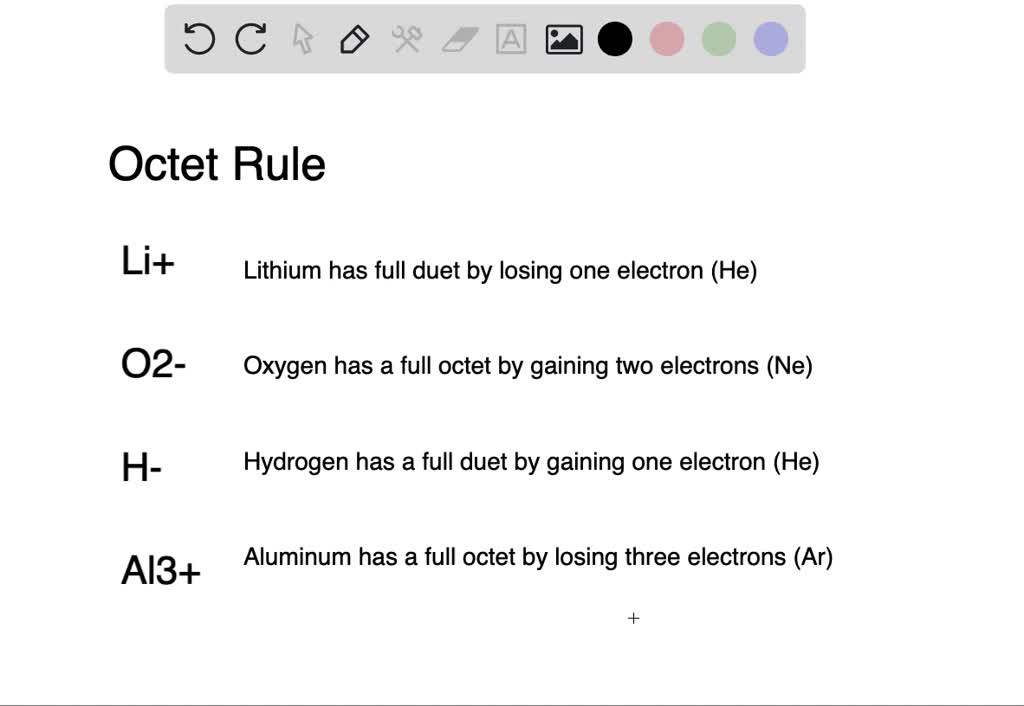
Solved Show How Each Chemical Change Obeys The Octet Rule A Hydrogen Forms H Hydride Ion B Aluminum Forms Al 3
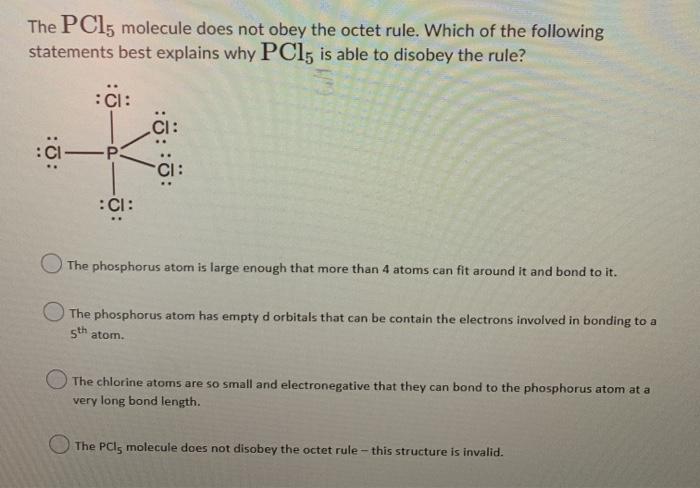
Solved The Pcl Molecule Does Not Obey The Octet Rule Which Chegg Com
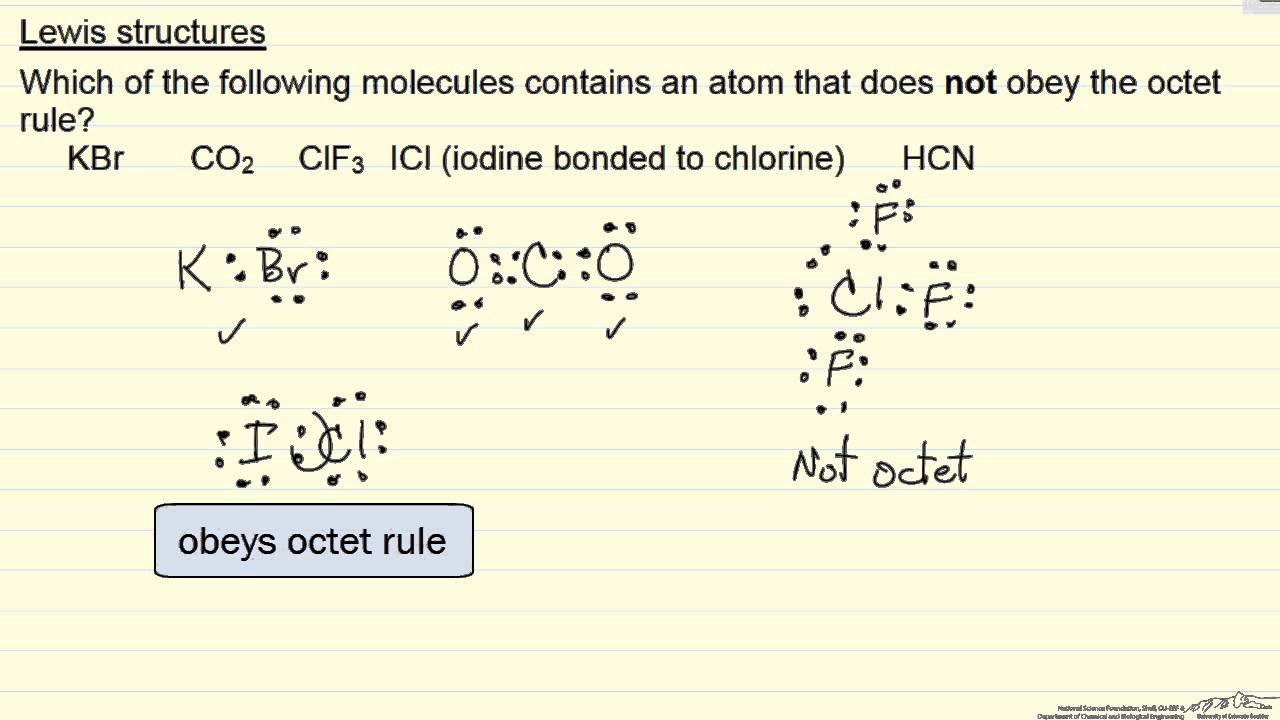
Lewis Structures Octet Rule Example Youtube

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube

Exceptions To The Octet Rule Ppt Video Online Download

Solved Which Of The Following Molecules Does Not Obey The Octet Rule A Mathrm Hcn B Mathrm Pf 3 C Mathrm Cs 2 D Mathrm No E None Of These

Octet Rule Song Octet Rule Covalent Bonding Electron Configuration

Comments
Post a Comment